Scientists unveil the hidden structures of materials and biomolecules using crystallography and cryo-electron microscopy (cryo-EM). However, as these disciplines face ever-increasing complications, machine learning has become a valuable ally.
In this post, we’ll look at the fascinating intersection of “Methods of Machine Learning for Crystallography and Cryo-EM.” Join us me as we investigate the revolutionary impact of artificial intelligence in unlocking the secrets of the atomic and molecular universes.
First of all, I want to ease into the topic and mention what exactly are the terms of crystallography and Cryo-Em, then we will investigate further where machine learning comes into the play.
Crystallography
Crystallography is the study of the arrangement of atoms in crystalline materials. Crystals are solids made up of atoms that are arranged in a repeating pattern to form a highly structured structure.
Because of this regular arrangement, materials have unique properties and behaviors, making crystallography vital for understanding the properties of many substances.
Scientists can examine the crystal lattice using techniques such as X-ray diffraction, giving crucial information on atom positions and bonding interactions. Crystallography is important in many fields, from materials science and chemistry to geology and biology. It helps with the development of new materials and the understanding of mineral properties.
It can even help us in deciphering the complicated structures of biological molecules such as proteins.
Cryo-EM (Cryo-Electron Microscopy)
Cryo-electron microscopy (Cryo-EM) is a sophisticated imaging technology that allows researchers to see the three-dimensional structures of biomolecules at atomic or near-atomic resolution.
Cryo-EM preserves biomolecules in their near-natural condition by fast freezing them in liquid nitrogen, as opposed to standard electron microscopy, which needs samples to be fixed, stained, and dehydrated.
This prevents ice crystal formation, preserving biological structure. Scientists can now see precise details of huge protein complexes, viruses, and cellular organelles, providing crucial insights into their functions and relationships.
Cryo-EM has transformed structural biology by allowing researchers to explore biological processes at previously unthinkable levels of detail. Its applications range from drug discovery and vaccine development to understanding illness molecular foundations.
Why They Are Important?
Cryo-EM and crystallography are crucial in furthering our understanding of the natural world.
Crystallography enables us to discover and comprehend the atomic arrangement in materials, allowing us to build novel compounds with specific qualities for a wide range of uses. Crystallography is essential in shaping our modern culture, from semiconductors used in electronics to medications used to treat ailments.
Cryo-EM, on the other hand, provides a fascinating view into the complicated mechanism of life. Scientists acquire insights into fundamental biological processes by viewing the architecture of biomolecules, allowing them to produce better medications, design targeted therapies, and efficiently combat infectious diseases.
Cryo-EM advancements open up new vistas in medicine, biotechnology, and our overall understanding of life’s building blocks.
Enhancing Structure Prediction and Analysis with Machine Learning in Crystallography
Machine learning has been incredibly helpful in crystallography, revolutionizing how scientists forecast and interpret crystal structures.
Algorithms can extract patterns and correlations from enormous datasets of known crystal structures, allowing for the quick prediction of new crystal structures with unparalleled precision.
For example, Thorn Lab researchers have proved the effectiveness of machine learning in forecasting crystal stability and formation energy, providing vital insights into the thermodynamic properties of materials.
This development not only accelerates the discovery of new materials but also the optimization of current ones, bringing in a new era of materials research with better qualities and functionalities.
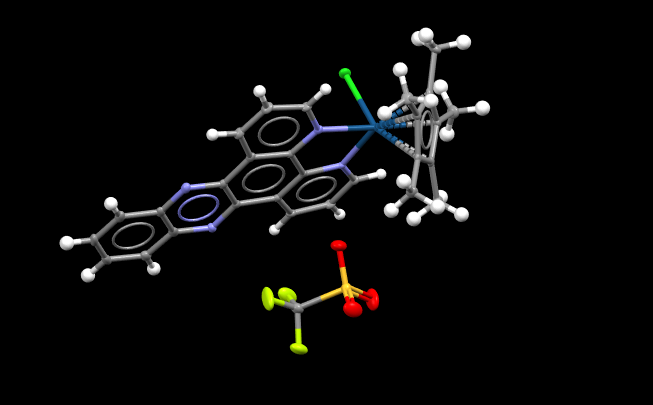
Image: An example of a crystal structure illustrated on Mercury software.
How Machine Learning Unveils the Cryo-EM?
Machine learning has opened up a new world of possibilities in cryo-electron microscopy (Cryo-EM), allowing scientists to delve deeper into the structural complexity of biomolecules.
Researchers can analyze massive volumes of cryo-EM data using novel technologies such as deep learning, reconstructing three-dimensional models of biological molecules with unparalleled clarity and accuracy.
This combination of machine learning with cryo-EM has allowed for the imaging of previously undecipherable protein structures, providing new insights into their activities and relationships.
The combination of these technologies holds enormous promise for drug discovery since it allows researchers to precisely target specific binding sites, leading to the creation of more effective medicines for a variety of disorders.
Machine Learning Algorithms for Accelerating Cryo-EM Data Analysis
Cryo-EM investigations generate detailed and massive datasets, which can be both a gift and a curse for researchers. However, machine learning methods have proven to be essential in the effective analysis and interpretation of cryo-EM data.
Scientists can use unsupervised learning approaches to automatically detect and classify various protein structures, reducing the time-consuming manual operations.
This method not only speeds up data analysis but also improves findings’ dependability by removing human biases in the interpretation of complicated structural data.
The incorporation of machine learning in Cryo-EM data analysis, as demonstrated in recent works, offers a way for a deeper knowledge of complicated biological processes and a more thorough examination of life’s molecular machinery.
Towards Hybrid Approaches: Bridging the Experiment-Computation Gap
Machine learning has the potential to bridge the gap between experimental data and computational models in crystallography and cryo-EM.
The combination of experimental data and machine learning techniques enables the development of precise predictive models, improving the reliability of structure determination and property estimate.
Transfer learning, a technique that applies knowledge learned in one area to another, appears as a significant tool for boosting the efficiency of crystallographic and Cryo-EM investigations in this context.
Hybrid techniques, which combine experimental insights with computer capacity, represent a cutting-edge option for solving challenging scientific challenges, promising to alter how we see and manipulate the atomic and molecular world.
Using Convolutional Neural Networks to Pick Particles in Cryo-EM
By giving high-resolution images of biological molecules, cryo-electron microscopy (Cryo-EM) has transformed the study of macromolecular structures.
However, particle picking, which entails recognizing and extracting individual particle images from Cryo-EM micrographs, has been a time-consuming and arduous task.
Researchers have made tremendous progress in automating this procedure with the use of machine learning, particularly convolutional neural networks (CNNs).
DeepPicker and Topaz-Denoise are two deep learning algorithms that enable fully automated particle selection in cryo-EM, considerably speeding up data processing and analysis.
CNN-based approaches have become critical in speeding Cryo-EM procedures and allowing researchers to focus on higher-level investigations by accurately detecting particles with high precision.
Optimization of Crystallography Using Predictive Modeling
The quality of diffraction data and crystallization outcomes can have a considerable impact on structure determination in macromolecular crystallography.
Artificial neural networks (ANNs) and support vector machines (SVMs) have been used successfully to optimize crystallization settings and forecast crystal diffraction quality. Predictive models produced by researchers aid in the design of experiments and enhance the success rate of crystallization trials.
These models can uncover patterns that lead to good outcomes by evaluating massive volumes of crystallization data, assisting researchers in producing high-quality crystals for subsequent X-ray diffraction tests. As a result, machine learning has become an indispensable tool for fast and targeted crystallographic testing.
Improving Cryo-EM Structural Recognition
Understanding the secondary structure of biological molecules using Cryo-EM density maps is critical for determining their functions and interactions.
Machine learning approaches, namely deep learning architectures such as graph convolutional and recurrent networks, have been used to locate secondary structure features in cryo-EM maps automatically.
These methods investigate local features in density maps, allowing for precise classification of secondary structural elements. Machine learning enables researchers to investigate complicated chemical structures and acquire insights into their biological activities by automating this labor-intensive process.
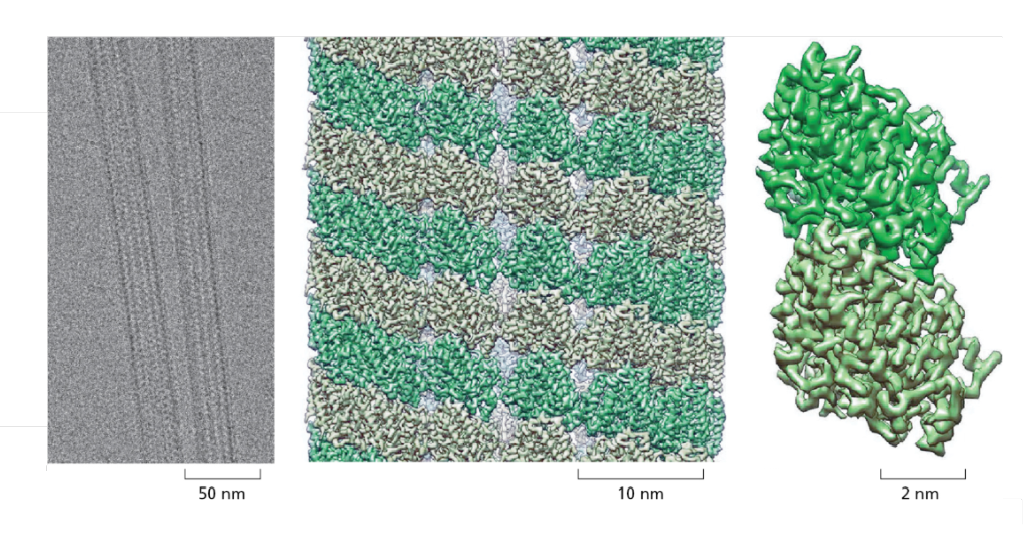
Image: Cryo-EM reconstitution of a structure
Crystallography Model Building and Validation Acceleration
Model construction and validation are key phases in macromolecular crystallography to assure structural model accuracy and reliability.
Machine learning technologies such as convolutional autoencoders and Bayesian models have been used to aid and improve these processes. AAnchor, for example, uses CNNs to recognize anchor amino acids in Cryo-EM density maps, which aids in automatic model development.
Bayesian machine learning models were also used to integrate X-ray diffraction data and assign space groups in small molecule electron density maps.
These advancements not only speed up structure determination but also provide more extensive assessments of model quality, resulting in more robust and reproducible research outputs.
Machine Learning’s Future in Structural Biology
As seen by the growing number of scientific publications, the integration of machine learning in cryo-EM and crystallography is constantly improving, providing a plethora of novel solutions and applications.
Machine learning promises to further transform the structural biology environment with the continuous development of powerful algorithms and the expansion of curated resources.
The synergy between machine learning and structural biology is paving the way for discoveries and insights into the atomic and molecular world, from quick structure determination to drug discovery and protein engineering.
The ongoing research on this fascinating topic inspires scientists to harness the power of AI and unlock the mysteries of life’s building blocks.
Conclusion
The incorporation of machine learning technologies into crystallography and cryo-electron microscopy has opened a new age in structural biology.
Machine learning has substantially expedited the pace of research and brought unparalleled insights into the atomic and molecular worlds, from automating arduous operations like particle selection to improving predictive modeling for crystallization and diffraction quality.
Researchers can now efficiently evaluate enormous volumes of data using convolutional neural networks and other advanced algorithms, instantly anticipating crystal structures and extracting valuable information from cryo-electron microscopy density maps.
These developments not only speed up experimental operations but also allow for a more in-depth study of biological structures and functions.
Finally, the convergence of machine learning and structural biology is altering the landscapes of crystallography and cryo-electron microscopy.
Together, these cutting-edge technologies are bringing us closer to a better understanding of the atomic and molecular worlds, promising game-changing breakthroughs in materials research, medication development, and the intricate machinery of life itself.
As we embrace this fascinating new frontier, the future of structural biology shines brightly with limitless possibilities and the ability to solve nature’s most difficult puzzles.





Leave a Reply